Carrying on from a recent thread about the potential dangers of amateurs installing unvented undersink water heaters incorrectly….. Click on this

As regular readers on here know I like rules of thumb that allow a quick decision of ‘its OK’ or ‘it needs checking properly’.
I am not a plumber, but for a quick look and see, for the expansion vessel on the water heater, a good start is expecting an expansion volume of about 10% of the volume of the heated water, rounding up, rather than rounding down, to the nearest catalogue size vessel available if you are replacing.
This over estimates if the incoming cold supply pressure is low, and underestimates if it is already at the high limit.
So a 2 liter expansion tank ought to handle anything a 20 litre or less water heater can throw at it. Less volume may be OK, but it depends how much over-pressure can be absorbed by stretching things that do not really appreciate it, such as making taps drip and forcing water back up the street main.
Mike.
PS
for heating the text book formula is
Vexp = V_system *e/(1-p1/p2)
where p1/p2 is the ratio of the cold to hot pressure, perhaps 0.7- 0.8
a moments thought shows this also has to be an approximation.
relative to 25 °C
Expansion Factor ‘e’ | Temperature °C |
0.0324 | 85 |
0.0359 | 90 |
0.0396 | 95 |
0.0434 | 100 |
edit
(reference )
Actually to do the sums properly, note the expansion of water is highly non-linear with temperature, the rate (fraction of extra volume per degree rise is about 3 times higher approaching boiling, than it is at room temp, less as it gets colder, while it inverts at 4°C and stops shrinking starts to expand going colder, an effect that increases sharply as freezing commences)
In terms of volume change in litres per litre per °C,
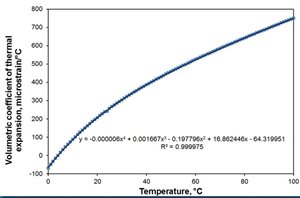
Or for those who like graphs (or indeed arbitrary curve fitting with no good physical justification, other than ‘excel does it’ ) , - parts per million volume expansion per degree temperature rise up the side, vs temperature. (source)
As regular readers on here know I like rules of thumb that allow a quick decision of ‘its OK’ or ‘it needs checking properly’.
I am not a plumber, but for a quick look and see, for the expansion vessel on the water heater, a good start is expecting an expansion volume of about 10% of the volume of the heated water, rounding up, rather than rounding down, to the nearest catalogue size vessel available if you are replacing.
This over estimates if the incoming cold supply pressure is low, and underestimates if it is already at the high limit.
So a 2 liter expansion tank ought to handle anything a 20 litre or less water heater can throw at it. Less volume may be OK, but it depends how much over-pressure can be absorbed by stretching things that do not really appreciate it, such as making taps drip and forcing water back up the street main.
Mike.
PS
for heating the text book formula is
Vexp = V_system *e/(1-p1/p2)
where p1/p2 is the ratio of the cold to hot pressure, perhaps 0.7- 0.8
a moments thought shows this also has to be an approximation.
relative to 25 °C
Expansion Factor ‘e’ | Temperature °C |
0.0324 | 85 |
0.0359 | 90 |
0.0396 | 95 |
0.0434 | 100 |
edit
(reference )
Actually to do the sums properly, note the expansion of water is highly non-linear with temperature, the rate (fraction of extra volume per degree rise is about 3 times higher approaching boiling, than it is at room temp, less as it gets colder, while it inverts at 4°C and stops shrinking starts to expand going colder, an effect that increases sharply as freezing commences)
In terms of volume change in litres per litre per °C,
Or for those who like graphs (or indeed arbitrary curve fitting with no good physical justification, other than ‘excel does it’ ) , - parts per million volume expansion per degree temperature rise up the side, vs temperature. (source)

We're about to take you to the IET registration website. Don't worry though, you'll be sent straight back to the community after completing the registration.
Continue to the IET registration site