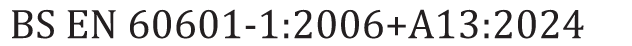BS7671 clause 710.415.2.1 requires that in each medical location of Group 1 activity, supplementary equipotential bonding connection points shall be installed.
It states: Supplementary equipotential bonding connection points for the connection of ME equipment shall be provided in each medical location, as follows: Group 1: a minimum of one per patient location.
For a consulting room in a Healthcare Centre, where patients will walk in and:
- the patient is only seeing a consultant for an appoint so is not in the room for any period of time
- the patient is typically in reasonably health and does not been supported/assisted in seeing the consultant
- there are no invasive procedures.
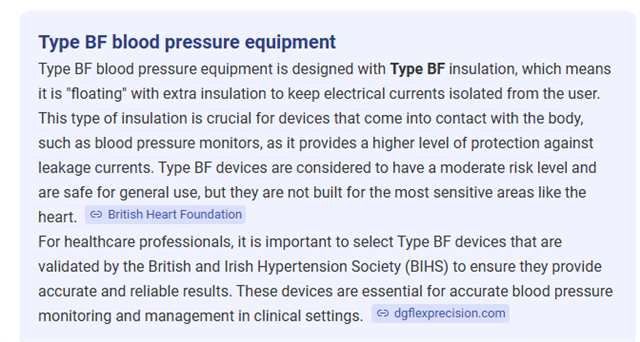
- the equipment being used is a blood pressure monitor, (type BF as defined in BS EN 6061)
Is the Supplementary equipotential bonding connection point needed - as there is no availabe connection point on the equipment to connect to the bonding connection point. ?
Is a deviation from the regulation permitted as long as the reason is stated on the test certificate?